Introduction
Colored (or chromophoric) dissolved organic matter (CDOM) is the part of organic matter that absorbs
light in the blue and UV part of the electromagnetic spectrum, staining water a “tea-like” yellow-brown
color. CDOM plays major roles in freshwater ecosystem processes, determining physical and chemical
conditions and water quality in freshwaters, and it is the most abundant DOM fraction in forested
watersheds with wetlands.
- Color Dissolved Organic Matter (CDOM): Background information (PDF)
- CDOM: Field-based studies (PDF)
- Measurement of CDOM Using Smartphone Cameras (PDF)
- Prediction of Photochemically Produced Reactive Intermediates in Surface Waters via Satellite Remote Sensing (PDF)
- CDOM and drinking water treatment (PDF)
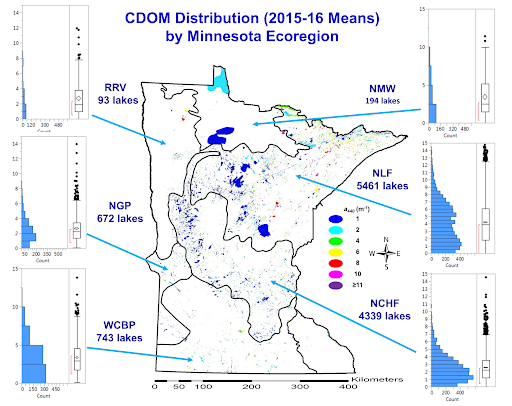
CDOM distribution in lakes across the NLF and NCHF ecoregions
We have examined CDOM distributions across two ecoregions, the Northern Lakes and Forests and North Central Hardwood Forests, that span the Upper Great Lakes states of Minnesota, Wisconsin and Michigan. Supported by a National Science Foundation grant this work included analysis of landscape conditions in watersheds that control CDOM levels in lakes.
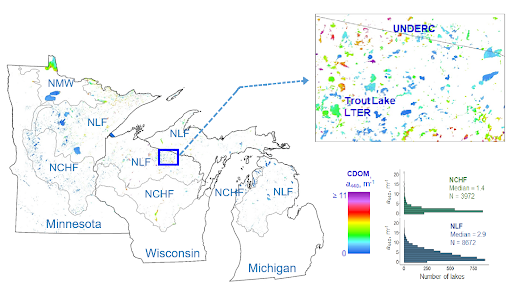
We began measuring CDOM by satellite imagery in 2014 (Olmanson et al. 2016) and expanded to statewide coverage in 2015 using Landsat 8 and Sentinel-2 (Olmanson et al. 2020). These data are being analyzed for statistical distributions and combined with earlier data for trend analyses. More detailed results for specific lakes are available on the LakeBrowser.